In an amazing new MIT Technology Review piece, Antonio Regalado discusses how, “Some scientists are taking a DIY coronavirus vaccine, and nobody knows if it’s legal or if it works.” It is another powerful example of how “citizen-science” and medical self-experimentation (or “biohacking”) is increasingly being used to improve health outcomes, enhance human capabilities, or fight against deadly diseases like COVID. Regalado reports that:
Nearly 200 covid-19 vaccines are in development and some three dozen are at various stages of human testing. But in what appears to be the first “citizen science” vaccine initiative, Estep and at least 20 other researchers, technologists, or science enthusiasts, many connected to Harvard University and MIT, have volunteered as lab rats for a do-it-yourself inoculation against the coronavirus. They say it’s their only chance to become immune without waiting a year or more for a vaccine to be formally approved.
Among those who’ve taken the DIY vaccine is George Church, the celebrity geneticist at Harvard University, who took two doses a week apart earlier this month. The doses were dropped in his mailbox and he mixed the ingredients himself.
Regalado notes that this is all happening despite legal and ethical questions:
By distributing directions and even supplies for a vaccine, though, the Radvac group is operating in a legal gray area. The US Food and Drug Administration (FDA) requires authorization to test novel drugs in the form of an investigational new drug approval. But the Radvac group did not ask the agency’s permission, nor did it get any ethics board to sign off on the plan.
Continue reading →
 Margaret Talbot has written an excellent New Yorker essay entitled, “The Rogue Experimenters,” which documents the growth of the D.I.Y.-bio movement. This refers to the organic, bottom-up, citizen science movement, or “leaderless do-ocracy” of tinkerers, as she notes. I highly recommend you check it out.
Margaret Talbot has written an excellent New Yorker essay entitled, “The Rogue Experimenters,” which documents the growth of the D.I.Y.-bio movement. This refers to the organic, bottom-up, citizen science movement, or “leaderless do-ocracy” of tinkerers, as she notes. I highly recommend you check it out.
As I noted in my new book on Evasive Entrepreneurs and the Future of Governance, “DIY health services and medical devices are on the rise thanks to the combined power of open-source software, 3D printers, cloud computing, and digital platforms that allow information sharing between individuals with specific health needs. Average citizens are using these new technologies to modify their bodies and abilities, often beyond the confines of the law.”
Talbot discusses many of the same examples I discuss in my book, including:
- the Four Thieves Vinegar collective, which devised instructions for building its own version of the EpiPen;
- e-nable, an international collective of thirty thousand volunteers, designs and 3-D-prints prosthetic hands and arms (and which has, more recently, distributed more than fifty thousand face shields in more than twenty-five countries.);
- GenSpace and other community biohacking labs; and
- Open Insulin and Open Artificial Pancreas System.
I like the way Talbot compares these movements to the hacker and start-up culture of the Digital Revolution: Continue reading →
In a new essay in The Dallas Morning News (“Licensing restrictions for health care workers need to be flexible to fight coronavirus“), Trace Mitchell and I discuss recent efforts to reform occupational licensing restrictions for health care workers to help fight the coronavirus. Trace and I have written extensively about the need for licensing flexibility over the past couple of years, but it is needed now more than ever. Luckily, some positive reforms are now underway.
We highlight efforts in states like Massachusetts and Texas to reform their occupational licensing rules in response to the crisis, as well as federal reforms aimed at allowing reciprocity across state lines. We conclude by noting that:
It should not take a crisis of this magnitude for policymakers to reconsider the way we prevent fully qualified medical professionals from going where they are most needed. But that moment is now upon us. More leaders would be wise to conduct a comprehensive review of regulatory burdens that hinder sensible, speedy responses to the coronavirus crisis.
If nothing else, the relaxation of these rules should give us a better feel for how necessary strict licensing requirements truly are. Chances are, we will learn just how costly the regulations have been all along.
Read the entire piece
here.
 My professional life is dedicated to researching the public policy implications of various emerging technologies. Of the many issues and sectors that I cover, none are more interesting or important than advanced medical innovation. After all, new health care technologies offer the greatest hope for improving human welfare and longevity. Consequently, the public policies that govern these technologies and sectors will have an important bearing on just how much life-enriching or life-saving medical innovation we actually get going forward.
My professional life is dedicated to researching the public policy implications of various emerging technologies. Of the many issues and sectors that I cover, none are more interesting or important than advanced medical innovation. After all, new health care technologies offer the greatest hope for improving human welfare and longevity. Consequently, the public policies that govern these technologies and sectors will have an important bearing on just how much life-enriching or life-saving medical innovation we actually get going forward.
Few people are doing better reporting on the intersection of advanced technology and medicine — as well as the effects of regulation on those fields — than my Mercatus Center colleague Jordan Reimschisel. In a very short period of time, Jordan has completely immersed himself in these complex, cutting-edge topics and produced a remarkable body of work discussing how, in his words, “technology can merge with medicine to democratize medical decision making, empower patients to participate in the treatment process, and promote better health outcomes for more patients at lower and lower costs.” He gets deep into the weeds of the various technologies he writes about as well as the legal, ethical, and economic issues surrounding each topic.
I encouraged him to start an ongoing compendium of his work on these topics so that we could continue to highlight his research, some of which I have been honored to co-author with him. I have listed his current catalog down below, but jump over to this Medium page he set up and bookmark it for future reference. This is some truly outstanding work and I am excited to see where he goes next with topics as wide-ranging as “biohackerspaces,” democratized or “personalized” medicine, advanced genetic testing and editing techniques, and the future of the FDA in an age of rapid change.
Give Jordan a follow on Twitter (@jtreimschisel) and make sure to follow his Medium page for his dispatches from the front lines of the debate over advanced medical innovation and its regulation.
Continue reading →
In theory, the Food & Drug Administration (FDA) exists to save lives and improve health outcomes. All too often, however, that goal is hindered by the agency’s highly bureaucratic, top-down, command-and-control orientation toward drug and medical device approval.
Today’s case in point involves families of children with diabetes, many of whom are increasingly frustrated with the FDA’s foot-dragging when it comes to approval of medical devices that could help their kids. Writing today in The Wall Street Journal, Kate Linebaugh discusses how “Tech-Savvy Families Use Home-Built Diabetes Device” to help their kids when FDA regulations limit the availability of commercial options. She documents how families of diabetic children are taking matters into their own hands and creating their own home-crafted insulin pumps, which can automatically dose the proper amount of proper amount of the hormone in response to their child’s blood-sugar levels. Families are building, calibrating, and troubleshooting these devices on their own. And the movement is growing. Linebaugh reports that:
More than 50 people have soldered, tinkered and written software to make such devices for themselves or their children. The systems—known in the industry as artificial pancreases or closed loop systems—have been studied for decades, but improvements to sensor technology for real-time glucose monitoring have made them possible.
The Food and Drug Administration has made approving such devices a priority and several companies are working on them. But the yearslong process of commercial development and regulatory approval is longer than many patients want, and some are technologically savvy enough to do it on their own.
Linebaugh notes that this particular home-built medical project (known as OpenAPS), was created by Dana Lewis, a 27-year-old with Type 1 diabetes in Seattle. Linebaugh says that: Continue reading →
 As “software eats the world,” the reach of the Digital Revolution continues to expand to far-flung fields and sectors. The ramifications of this are tremendously exciting but at times can also be a little bit frightening.
As “software eats the world,” the reach of the Digital Revolution continues to expand to far-flung fields and sectors. The ramifications of this are tremendously exciting but at times can also be a little bit frightening.
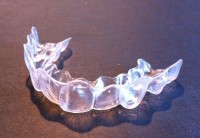
Consider this recent Washington Post headline: “A College Kid Spends $60 to Straighten His Own Teeth. What Could Possibly Go Wrong?” Matt McFarland of the Post reports that, “A college student has received a wealth of interest in his dental work after publishing an account of straightening his own teeth for $60.” The student at the New Jersey Institute of Technology, “had no dentistry experience when he decided to create plastic aligners to improve his smile,” but was able to use a 3D printer and laser scanner on campus to accomplish the job. “After publishing before-and-after pictures of his teeth this month, [the student] has received hundreds of requests from strangers, asking him to straighten their teeth.”
McFarland cites many medical professionals who are horrified at the prospect of patients taking their health decisions into own hands and engaging in practices that could be dangerous to themselves and others. Some of the licensed practitioners cited in the story come across as just being bitter losers as they face the potential for the widespread disintermediation of their profession. After all, they currently charge thousands of dollars for various dental procedures and equipment. Thanks to technological innovations, however, those costs could soon plummet, which could significantly undercut their healthy margins on dental services and equipment. On the other hand, these professionals have a fair point about untrained citizens doing their own dental work or giving others the ability to do so. Things certainly could go horribly wrong.
This is another interesting case study related to the subject of a forthcoming Mercatus paper as well as an upcoming law review article on 3D printing of mine, both of which pose the following question: What happens when radically decentralized technological innovation (such as 3D printing) gives people a de facto “right to try” new medicines and medical devices? Continue reading →
I’ve been thinking about the “right to try” movement a lot lately. It refers to the growing movement (especially at the state level here in the U.S.) to allow individuals to experiment with alternative medical treatments, therapies, and devices that are restricted or prohibited in some fashion (typically by the Food and Drug Administration). I think there are compelling ethical reasons for allowing citizens to determine their own course of treatment in terms of what they ingest into their bodies or what medical devices they use, especially when they are facing the possibility of death and have exhausted all other options.
But I also favor a more general “right to try” that allows citizens to make their own health decisions in other circumstances. Such a general freedom entails some risks, of course, but the better way to deal with those potential downsides is to educate citizens about the trade-offs associated with various treatments and devices, not to forbid them from seeking them out at all.
The Costs of Control
But this debate isn’t just about ethics. There’s also the question of the costs associated with regulatory control. Practically speaking, with each passing day it becomes harder and harder for governments to control unapproved medical devices, drugs, therapies, etc. Correspondingly, that significantly raises the costs of enforcement and makes one wonder exactly how far the FDA or other regulators will go to stop or slow the advent of new technologies.
I have written about this “cost of control” problem in various law review articles as well as my little Permissionless Innovation book and pointed out that, when enforcement challenges and costs reach a certain threshold, the case for preemptive control grows far weaker simply because of (1) the massive resources that regulators would have to pour into the task on crafting a workable enforcement regime; and/or (2) the massive loss of liberty it would entail for society more generally to devise such solutions. With the rise of the Internet of Things, wearable devices, mobile medical apps, and other networked health and fitness technologies, these issues are going to become increasingly ripe for academic and policy consideration. Continue reading →
Earlier this week I posted an essay entitled, “Global Innovation Arbitrage: Commercial Drones & Sharing Economy Edition,” in which I noted how:
Capital moves like quicksilver around the globe today as investors and entrepreneurs look for more hospitable tax and regulatory environments. The same is increasingly true for innovation. Innovators can, and increasingly will, move to those countries and continents that provide a legal and regulatory environment more hospitable to entrepreneurial activity.
That essay focused on how actions by U.S. policymakers and regulatory agencies threatened to disincentivize homegrown innovation in the commercial drone and sharing economy sectors. But there are many other troubling examples of how America risks losing its competitive advantage in sectors where we should be global leaders as innovators looks offshore. We can think of this as “global innovation arbitrage,” as venture capitalist Marc Andreessen has aptly explained:
Think of it as a sort of “global arbitrage” around permissionless innovation — the freedom to create new technologies without having to ask the powers that be for their blessing. Entrepreneurs can take advantage of the difference between opportunities in different regions, where innovation in a particular domain of interest may be restricted in one region, allowed and encouraged in another, or completely legal in still another.
One of the more vivid recent examples of global innovation arbitrage involves the well-known example of 23andMe, which sells mail-order DNA-testing kits to allow people to learn more about their genetic history and predisposition to various diseases. Continue reading →

 Margaret Talbot has written an excellent New Yorker essay entitled, “
Margaret Talbot has written an excellent New Yorker essay entitled, “

 The Technology Liberation Front is the tech policy blog dedicated to keeping politicians' hands off the 'net and everything else related to technology.
The Technology Liberation Front is the tech policy blog dedicated to keeping politicians' hands off the 'net and everything else related to technology.