 As “software eats the world,” the reach of the Digital Revolution continues to expand to far-flung fields and sectors. The ramifications of this are tremendously exciting but at times can also be a little bit frightening.
As “software eats the world,” the reach of the Digital Revolution continues to expand to far-flung fields and sectors. The ramifications of this are tremendously exciting but at times can also be a little bit frightening.
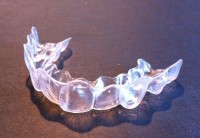
Consider this recent Washington Post headline: “A College Kid Spends $60 to Straighten His Own Teeth. What Could Possibly Go Wrong?” Matt McFarland of the Post reports that, “A college student has received a wealth of interest in his dental work after publishing an account of straightening his own teeth for $60.” The student at the New Jersey Institute of Technology, “had no dentistry experience when he decided to create plastic aligners to improve his smile,” but was able to use a 3D printer and laser scanner on campus to accomplish the job. “After publishing before-and-after pictures of his teeth this month, [the student] has received hundreds of requests from strangers, asking him to straighten their teeth.”
McFarland cites many medical professionals who are horrified at the prospect of patients taking their health decisions into own hands and engaging in practices that could be dangerous to themselves and others. Some of the licensed practitioners cited in the story come across as just being bitter losers as they face the potential for the widespread disintermediation of their profession. After all, they currently charge thousands of dollars for various dental procedures and equipment. Thanks to technological innovations, however, those costs could soon plummet, which could significantly undercut their healthy margins on dental services and equipment. On the other hand, these professionals have a fair point about untrained citizens doing their own dental work or giving others the ability to do so. Things certainly could go horribly wrong.
This is another interesting case study related to the subject of a forthcoming Mercatus paper as well as an upcoming law review article on 3D printing of mine, both of which pose the following question: What happens when radically decentralized technological innovation (such as 3D printing) gives people a de facto “right to try” new medicines and medical devices? In one sense, decentralized, democratized innovation of this sort presents us with an exciting new world of possibilities. On the other hand, we know that when average citizens take their health into their own hands, the results could be disastrous. The question is, what do want policymakers to do about it? Ban 3D printers? Restrict the distribution of 3D printed blueprints freely shared online? Try to license average users? Or regulate the materials used to make these medical devices?
For the reasons I suggest in my forthcoming paper, none of these options are likely to work very well in practice. It will prove too complex and costly to employ top-down, command-and-control regulation in a world of such decentralized innovation. Moreover, many people will also find it highly offensive if the government takes steps to limit their personal autonomy and ability to self-treat themselves at a much lower cost than our currently health care system typically demands for similar treatments. The example in McFarland’s story is quite powerful in that regard because, as it makes clear, even young kids could be engaging in this sort of innovation and self-experimentation, at greatly reduced cost to themselves or their families. Again, this is both wonderful and a little bit scary.
The best hope, I argue in my forthcoming papers, lies in improved risk education. The goal should be to help create a more fully-informed citizenry that is empowered with more and better information about relative risk trade-offs. The Food & Drug Administration already engages in various product labeling efforts as well as public education campaigns and strategies. But this has always been a secondary mission for the agency, which has instead focused on trying to preemptively guarantee the safety and efficacy of drugs and devices. And much of the “education” the FDA does is basically explaining to companies and the public how to comply with its voluminous body of regulation.
This is going to have to change, and change quickly. Going forward, the FDA will likely have to reorient its focus in this way to cope with the rapidly evolving universe of not just mobile medical apps and 3D-printed technologies, but also all the wearable technologies that are part of the larger Internet of Things. For example, the FDA recently released a guidance document for “Management of Cybersecurity in Medical Devices,” encouraging innovators and other stakeholders to address security vulnerability in a collaborative, flexible fashion. This same model could be applied to 3D printing and many other new technologies. As I continue on to note in my forthcoming paper:
Guidance documents should be crafted that suggest various best practices for developers as well as risk education and communication messaging for the general public. The downside of such guidance documents, however, is that they leave unanswered the question of exactly what regulatory authority the agency might bring to bear against companies who are found to violate the “voluntary” principles or best practices in the documents. On the other hand, those guidance documents are usually superior to the alternative path of overly-rigid, top-down, preemptive controls on innovation. Congress should monitor the FDA’s use of such guidance documents closely to ensure that the agency does not abuse its broad regulatory discretion through arbitrary guidance actions.
My forthcoming papers also suggests that other non-governmental bodies will need to play a more active role in this risk education process and help explain safe and sensible uses of new technologies to the public, especially kids. And product developers will need to step-up their “safety-by-design” efforts to try to make sure that the products they release into the wild are as safe as possible. Of course, as with other general purpose technologies (like computers and smartphones), there is only so much that can be done preemptively to make sure devices like 3D printers are “safe and secure” out of the box. The reality is that, the more open and generative a new technology or platform, the harder it is to preemptively design it in such a way to foresee and limit all its uses–for better or for worse.
We live in exciting times, but serious risks exist when radical technological decentralization places tools and capabilities in the hands of average citizens. The goal of public policy should not be to retard the development or distribution of all these wonderful new tools, but instead to redouble efforts to education citizens about proper and improper uses of them.

 The Technology Liberation Front is the tech policy blog dedicated to keeping politicians' hands off the 'net and everything else related to technology.
The Technology Liberation Front is the tech policy blog dedicated to keeping politicians' hands off the 'net and everything else related to technology.